2Department of Radiation Oncology, Kalyan Singh Super Specialty Cancer Institute, Lucknow-India DOI : 10.5505/tjo.2024.4280
Summary
OBJECTIVETo study the dosimetric characteristics and treatment plan complexity of Intensity-Modulated Radiotherapy (IMRT) and RapidArc (RA) techniques for Flattening Filter (FF) and Flattening Filter-Free (FFF) beams in the treatment of cervical cancer.
METHODS
A cohort comprising twenty post-operative cervical cancer patients was selected for this study. Four distinct
sets of treatment plans were generated utilizing both RA and IMRT techniques employing FFF and
FF beams. The dosimetric parameters were subjected to a comprehensive comparison, encompassing
considerations such as the coverage of the Planning Target Volume (PTV), Conformity Index, Homogeneity
Index, Heterogeneity Index, Gradient Index, Organ at Risk doses, and Peripheral doses.
RESULTS
The dose-volume parameters exhibited a significant difference in V95 between RA_FF and FFF plans.
However, V98 demonstrated a higher percentage of coverage with FF beams for both IMRT and RA planning
techniques (p<0.01). IMRT and RA plans resulted in a percentage reduction in V45 for the bladder
and rectum with the FFF beam. Furthermore, the FFF beam showed a significant increase in MUs and
a significant reduction in V30% for the femoral head for both IMRT and RA plans. No difference was
observed in normal tissue sparing with the FFF beam for both techniques.
CONCLUSION
Dosimetrically, FF and FFF beam plans exhibit comparable target coverage and OAR sparing for postoperative
cervical carcinoma using both IMRT and RA techniques. However, in terms of plan quality,
RA_FFF plans demonstrate a superior coverage index, conformity, and better sparing of normal tissue
compared to IMRT_FFF, except for homogeneity.
Introduction
Cervical cancer is one of the most prevalent malignant tumors in women. Globally, it stands as the fourth leading cause of cancer-related deaths in women.[1] A considerable majority of cases occur in lower and middle-income countries, primarily due to the absence of widespread implementation of population-based cancer screening initiatives and human papillomavirus (HPV) vaccination.[2] In the initial stages, cervical cancer is commonly treated with surgical intervention, while radiotherapy and chemotherapy take precedence in intermediate and advanced stages. Concurrent chemoradiotherapy is globally recommended as the primary treatment approach for patients with International Federation of Gynecology and Obstetrics (FIGO) IB-IVA cancer when opting for definitive radiotherapy (RT). Radiotherapy is the preferred strategy for definitive and postoperative management of cervical and endometrial cancer.[3]Traditional radiotherapy methods, such as the fourfield box approach and front-to-back penetrating irradiation, have been widely used. However, they are associated with significant adverse effects on the gastrointestinal, urinary, and hematopoietic systems. In contrast, threedimensional conformal radiation therapy (3DCRT) has more advantages than conventional therapy. It tailors the radiation beam according to the primary target, resulting in more precise target coverage while adhering to organat- risk constraints. Intensity-Modulated Radiotherapy (IMRT) and Volumetric-Modulated Arc Therapy (VMAT) are two contemporary approaches to delivering precise radiation doses to tumors, minimizing exposure to surrounding healthy tissues.[4] Intensity-modulated radiotherapy is effective in preserving surrounding normal tissues and organs together and provides a high dose to the target. The major disadvantage of IMRT is that it consumes longer treatment time and uses many fixed beam angles and monitor units (MU).[5]
Further, with technological advancement, intensity- modulated radiation therapy with image-guided treatment delivery (IG-IMRT) has been commonly employed due to its low acute toxicity profile, that is, acute grade II gastrointestinal (GI) toxicity of 60% with intensity-modulated radiation therapy (IMRT) versus 91% with 3DCRT.[6,7] On the contrary, treatment planning with the Rapid Arc (RA) (Varian Medical Systems, Palo Alto, CA) technique, which usually employs using one or more arcs by changing dose rate, multi-leaf collimator location, and gantry speed, integrates to decrease the number of MU and shortens treatment time compared to IMRT, resulting in maximal dosage to target from all angles while preserving normal tissues.[8]
VMAT and IMRT planning with FF beam is associated with certain drawbacks, such as prolonged delivery time, reduced treatment dose rate, decreased photon intensity, and increased treatment dose scattering. Therefore, flattening filter-free (FFF) beams were intended to decrease the long delivery treatment time since removing the flattening filter raises the dose rate by a factor of two to four.[9] A reduction of the treatment time reduces the probability of intrafraction motion of the target and organs at risk, which has been demonstrated to be not negligible for the treatment of prostate cancer.[10] Moreover, FFF beams differ significantly from traditional photon beams in several ways. In addition to having a distinct photon energy spectrum and varied headscatter characteristics, they also have a different beam profile and a higher dose rate. As a result, FFF beams have unique beam characteristics such as a sharper penumbra, less head scatter, lower out-of-field dosage, and dosimeter response such as higher ion recombination.
Few studies have been conducted on the dosimetric effects of the FFF beam on RapidArc planning for cervical cancer. At the same time, faster treatments could have a clinical impact on cervical cancer patients in terms of comfort on the treatment table, immobility, and minimization of internal organ status changes, such as bladder or rectum filling changes over time, as well as the reduction of intra-fractional patient motion.[11-13]
Moreover, the previous studies were driven by the anticipation that variations in nominal energy and penumbra of Flattening Filter-Free (FFF) beams might affect the dosimetric outcomes for this particular deepseated treatment site. Changes in secondary build-up could potentially influence target coverage and the sparing of organs at risk (OAR). Hence, the objective of this study is to identify the optimal treatment modality for post-hysterectomy cervical cancer treatment. This involves a comprehensive analysis and comparison of plan quality, utilizing a flattening filter-free beam in conjunction with Intensity Modulated Radiation Therapy (IMRT) and RapidArc (RA) procedures, assessed through various dosimetric indices.
Methods
Patient SelectionTwenty consecutive patients with histologically proven locally advanced cervical cancer were retrospectively included in this planning study. The carcinoma cases were graded according to the FIGO 2018 classification. The sample size for our study was determined through a power analysis, referencing Deng et al.[14]'s study. Deng et al.[14] reported a power of 100% with D2 values (conformal radiotherapy (CRT) =650.8±48.9, IMRT =4907.0±47.9, VMAT =4962.2±22.5). Pairwise statistical differences were observed (CRT vs. IMRT, p<0.001; CRT vs. VMAT, p<0.001; IMRT vs. VMAT, p=0.002), with alpha (α=0.05) values obtained from the same study involving 15 patients. Therefore, we conducted a power analysis, determining a sample size of 20 patients to enhance the robustness of our study.
Simulation
The simulation was performed using CT-Sim (64 slices,
Philips Ingenuity) in a supine position. Standard bladder
protocol was maintained for all patients during
simulation and treatment. A contrast-enhanced computed
tomography (CECT) simulation was acquired
from L2 to mid-thigh with a slice thickness of 3.0 mm
for all planning CT images.
Contouring and Prescription of Target Volumes
The Clinical Target Volume (CTV) and Organs at Risk
(OARs) of each patient were contoured by an experienced
oncologist. The corresponding planning target volume
(PTV) was generated by symmetrically expanding 7.0
mm from CTV. The OARs included the rectum, bladder,
femur heads, and bowel in this study. In addition, to
improve the target dose conformity, the assistance organ
Body-PTV (B-P) was defined as the body volume in the
CT data set minus the PTV, leaving a 0.3 cm gap. Furthermore,
B-P was used in all RapidArc and IMRT optimization
to standardize the optimized constraints. The
prescribed dose to the target was 45 Gy in 25 fractions.
Treatment Planning
All the RapidArc and IMRT plans were generated using
the Eclipse (v15.6 Varian Medical Systems, Palo
Alto, CA, USA) treatment planning system. RapidArc
plans were created using the dual arc (181-179 were set
in the clockwise direction, and 179?181 were set in the
counterclockwise direction), and IMRT plan seven fixed
angles (51°, 102°, 151°, 202°, 251°, 302°, and 351°) were
used with jaw tracking using FF, and FFF 6MV beam and
Photon optimizer (PO) (Version 15.6.06, Varian Medical
System) was selected for inverse optimization by physical
and biological objectives. Hence, the physical constraints
as Upper, Lower, and Mean objectives were used to limit
the dose level in a defined portion of the structure volume,
define a minimum dose level that a particular target
volume should receive, and define the mean dose
that should not be exceeded for the structure. In addition,
the biological objective mainly used for OARs was
Upper gEUD, where the parameter "a" can vary from
+0.1 to +40. Each set of plan doses was calculated using
the Anisotropic Analytical Algorithm (AAA) (Version
15.6.06, Varian Medical System) with a 2.5 mm dose
grid resolution. Hence, the current study generated four
plans (RA_FF, RA_FFF, IMRT_FF, and IMRT_FFF) for each patient. The Varian TrueBeam accelerator equipped
with 120 leaves Millennium multi-leaf collimator (M120,
MLC) was used to develop all RA and IMRT plans with a
maximum dose rate of 600 MU/min and 1400 MU/min
for FF and FFF photon beams, respectively.
Dosimetric Evaluation
The requirement of a conformal and homogeneous
dose to the tumor is achieved in our case with no overdose
or underdose. So, the quality of treatment plans is
assured. IMRT and RA plans were quantitatively evaluated
using dose-volume histogram (DVH) curve analysis.
Various dosimetric metrics were evaluated using
cumulative dose-volume histogram (DVH). Isodose
distribution and dose-volume metrics were evaluated
for the PTV volume received by 95% and 98% of the
prescribed (V95, V98), near max (D2%), near min (D98%),
Maximum Dose (Dmax), Minimum Dose (Dmin), Mean
Dose (Dmean), D20, D50, D80 and V107(cc).
Plan Evaluation Indices
The Homogeneity Index (HI) used in this study is the
ratio of maximum dose (Dmax) to prescription dose
(PD). It is defined as the ratio of the maximum dose
delivered to the target volume to the prescribed dose as
per the RTOG protocol. If the value of HI is closer to 1,
it indicates better homogeneity.[15]
Target volume coverage (C) is the ratio of Dmin to PD. The plan is acceptable if TV covers 90% of the prescription isodose.[16]
The Conformity Index (CI) provides a reliable method for quantifying the degree of conformity based on isodose surfaces and volumes. It was calculated using the formula as reported in the RTOG 90?05 protocol. It is defined as the prescription isodose volume (PIV) that completely envelops the target volume (TV).[17]
The Gradient Index (GI) measures the shallowness or steepness of dose fall-off in tumor volume. GI is defined as the volume of PD to the 50% isodose volume of PD. A lower GI ratio indicates greater dose fall-off and better plan conformity.[18]
Akpati et al.[19] proposed a unified dosimetry index (UDI) that integrates contributions from all four above dosimetric components. It is considered an efficient tool for defining an ideal plan, with a value of one for an ideal treatment plan.
UDI = Coverage (C) × CI × HI × GI
The Dose Heterogeneity Index (DHI) is computed to find the dose homogeneity inside the target volume. This index is defined as follows:[20]
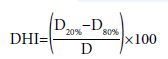
D20 and D80 represent the dose covering 20% and 80% of the target volume, respectively, and D is the prescription dose. According to the definitions of D20 and D80, D20 is always greater than or equal to D80. Therefore, a lower index reflects a smaller difference between the doses covering 20% and 80% of the target volume and indicates better dose homogeneity.
The low Gradient Index (LGI) and High-Gradient Index (HGI) were calculated using the formula below. Low and high gradient indices were calculated using the following formula:
Low Gradient Index (LGI)=V25% PID / V50% PID High Gradient Index (HGI)=V50% PID/V90% PID V25%, V50%, and V90% were volumes receiving 25%, 50%, and 90% of the prescription isodose dose (PID), respectively.
The OAR dose was compared using the following parameters: For the bladder and rectum, dosimetric parameters were analyzed using volume dose received by 30%, 40%, and 45% of the organ volume (V30, V40, V45) mean Dose (Dmean) and near maximum dose (D2cc). The dosimetric parameters V30(%) and D2cc were assessed in the femoral heads. V40 and V45 (volume in cc receiving 40 and 45 Gy) dose volumes were used to analyse the bowel. Additionally, the study considered parameters such as the body-PTV mean dose, low dose volumes (V1, V2, V3, V4, and V5), intermediate-dose volumes (V10, V20, V30 and V40), and monitor units (MU).
Statistical Analysis
The dosimetric difference between IMRT and RA plans
was analyzed using the Statistical Package for the Social
Sciences (version 23; IBM Corp., Armonk, NY, USA)
in terms of the mean, standard deviation, and P-values.
The independent paired t-test with a confidence interval
limit of 95% was performed to assess the dosimetric
endpoints for the target and OARs. P-values of less
than 0.05 were used to denote statistical significance.
Results
The mean volume of PTV, bladder, rectum, bowel, RTFH, and LTFH of all 20 patients were 1103.28±89.72 cm³, 163.57±78.04 cm³, 62.14±26.44 cm³, 1962.88±781.43 cm³, 97.26±10.70 cm³, and 97.01±12.63 cm³ [mean± standard deviation (SD)].Clinically acceptable treatment plans were created using the RA and IMRT techniques for all patients. Qualitative and quantitative analyses were performed on dose distribution created for RA and IMRT plans. The data were derived from cumulative DVH data for each treatment plan.
Table 1, Table 2, Table 3, and Table 4 summarize the planning target volume and OAR dose of IMRT and RA plans of FF and FFF beam plans.
Figures 1-4 represent the comparison between IMRT and RA plans for FF and FFF in terms of PTV coverage, bladder and rectum DVH parameters, and various plan quality indices.
Figure 5 represents the dose fall-off in the log-log plot between Dose (1 Gy to 40 Gy) in the BODY-PTV region for RA and IMRT plans for the FF and FFF techniques.
Figures 6, 7 show the isodose distribution in transverse, coronal, and sagittal planes for one patient planned with IMRT and RA techniques with FF and FFF beams. In the figures, "ns" denotes non-significant (p>0.05), while "*" signifies p?0.05.
The DVH comparison between IMRT and RA plans for FF and FFF plans is shown in Figures 8, 9.
PTV Dose Distributions and Evaluations
As depicted in Figures 6, 7 of isodose distribution and
Figures 8, 9 of DVH, no difference in V95 dose distribution
was observed between IMRT FF and FFF plans. In
contrast, a significant difference was observed between
RA_FF and FFF plans, depicted in Figure 1. Moreover,
V98 showed a higher percentage of dose distribution in
plans with FF beams for both IMRT and RA planning
techniques, with a significant difference observed between
them (p<0.01).
Furthermore, there was a significant reduction in D50 observed in IMRT_FFF plans. Conversely, an increase in D50 was observed (p=0.18) with RA_FFF. Additionally, IMRT_FFF plans showed a decrease in Dmax (p=0.14), whereas RA_FFF plans demonstrated a significant increase in Dmax inside the PTV volume.
Plan Quality Indices
IMRT_FF and IMRT_FFF plans showed a homogeneous
plan with a 6 MV photon beam, but a significant
difference in homogeneity was observed with
RA_FFF plans. Furthermore, there was a significant difference of 14.26% (p<0.01) in DHI with RA_FFF
plans, whereas with IMRT_FFF, the difference was only 4.27%, which was insignificant. A highly conformal
plan was found with RA_FF and RA_FFF techniques. However, a significant difference was observed
with IMRT plans for FF and FFF.
The evaluation of UDI, LGI, and HGI indices revealed a significant difference in UDI between IMRT and RA plans utilizing the FFF beam. However, all the plans achieved similar plan quality indices in their respective techniques. Additionally, no differences were observed in LGI and HGI indices among plans using the FFF beam, IMRT, and RA.
Dose Sparing of the OARs
Bladder: There was a significant increase in V30, V40,
and Mean Dose (Dmean) observed with the IMRT_FFF
beam; however, a reduction in V45 was found with
IMRT_FFF (p<0.05). Furthermore, no significant difference
was observed in the near-max dose (D2cc). For
RA_FFF, a decrease in V45 was found (p=0.07), and a
significant increase in D2cc was observed (p<0.01).
Rectum: A significant increase in V30, V40, and Mean Dose (Dmean) was found with IMRT_FFF plans. However, no difference was observed in V45 and D2cc. Furthermore, no significant difference was found between RA_FF and RA_FFF plans; a decrease in V45 was found with RA_FFF plans.
A large percentage reduction in V45 was observed with IMRT plans compared to RA for both the bladder and rectum, as shown in Figures 2, 3.
Bowel: The RA_FFF plans showed a reduction in V40, but no improvement was observed in V45. On the contrary, there was a significant increase in V40 with the IMRT_FFF technique; however, a significant reduction was observed in V45. Moreover, a similar scenario was observed with RA_FFF, but these differences are not statistically significant.
RTFH & LTFH: In both femoral heads, no significant differences were found between IMRT_FF and IMRT_ FFF, except in V30 (p<0.05). However, a significant increase in femoral head dose was found with RA_FFF plans.
The total number of monitor units (MU) is important for assessing the low dose to normal tissue and treatment time. The present study observed a significant increase in monitor units with FFF beam plans for IMRT and RA techniques. Moreover, the percentage difference in the increase in MUs is less in RA (12.16%) plans compared to IMRT (32.46%) plans.
The quantitative analysis of Figure 5 showed that dose fall-off beyond the target region was similar for all the datasets. Furthermore, we have taken ln(D) vs. ln(1/V) to evaluate the rate of dose fall-off beyond PTV. The fall-off shows that for low dose volumes (V1, V2, V3, V4, and V5), the change in dose fall-off is similar, which continues till V10. However, a steep dose fall-off was observed with intermediate dose volumes (V20, V30 and V40), starting from V20, which clearly shows a steeper dose gradient found with RA plans with FF compared to FFF for V20 and V30 (p<0.01).
Discussion
In FFF beams, the softening of the photon energy spectra leads to the shift of the maximum dose to the surface, peak forward, and a smaller penumbra width, resulting in reduced dispersion from the unit head.[9,21] These characteristics have been very beneficial for the treatment of various tumors. Furthermore, in addition to inverse planning, computer optimization provides significant flexibility to effectively address FFF beams" non-uniform profile. The dosimetric advantages of FFF beams in the treatment of postoperative cervical cancer patients were investigated by comparing them directly with RA and IMRT techniques in the current study.FFF beam plans showed similar target coverage and increased bladder, rectum, and femoral head protection with RA techniques compared to IMRT. Furthermore, RA plans achieved less MU than IMRT. The RA_FFF plans showed a higher maximum dose to PTV, as presented by Dmax and D2, compared with IMRT_FFF for better target coverage. Furthermore, with both techniques, a significant reduction in V98 was found with the FFF beam, as shown in Figure 3. Similar results were found by Manna et al.[11] using RA_FFF dose distribution. On the contrary, Tamilarasu et al.[12] showed no difference in dose distribution between FF and FFF with IMRT except D50 D2%, and no significant difference was found in D98% and D95% of the PTV. However, in the current study, a significant reduction in D50 was observed with IMRT_FFF plans. Conversely, an increase in D50 was observed (p=0.18) with RA_FFF. This referred to the fact that a fixed-field IMRT has a limited number of radiation beams, leading to the omission of some ideal beam angles; the RA technique utilizes all the available degrees of freedom during optimization. This approach contributes to the generation of an optimal dose distribution, resulting in improved treatment plans.
In the current study, we have used many plan quality indices to qualitatively analyze the treatment plans generated by the FFF beam compared to FF for IMRT and RA techniques. The results are compared to find out the effective treatment plan for the treatment of postop cervical cancer patients. Our study showed that RA produces more conformal and homogeneous plans than IMRT. This was consistent with the report that more conformal and homogeneous plans using the RA technique for post-operative cervical cancer patients.[14]
However, there was no difference in the Gradient Index for RA and IMRT plans. With FFF, most of the quality indices showed non-significant variation, except CI with IMRT_FFF plans and HI with RA_FFF plans. In addition, a decrease in UDI value was observed with IMRT_FFF, as shown in Figure 4, although this is not statistically significant. Zhang et al.[22] showed that VMAT_FFF produces an inferior heterogeneity plan compared to VMAT_FF while keeping similar conformity in the modalities. Moreover, Treutwein et al.[23] found an inferior plan quality with IMRT_FFF in terms of both HI and CI compared to IMRT_FF. We quantified that the RA plans irradiated more dose to the left and right femoral heads compared to doses to the bladder, rectum, and small bowel in IMRT plans. However, the differences were not statistically significant. These differences indicated that the number of fields used for IMRT directly impacts the quality of the IMRT plan, as RA plans decreased the MU and delivery time reported in previous studies.[24,25]
For OAR, the doses are smaller for FF than for FFF; partly, this can be traced to a smaller part of the PTV receiving the minimum dose required by the objectives. FFF for both bladder and rectum showed a decrease in dose for V45; however, this is not statistically significant. Furthermore, an increase in dose in dosimetric parameters (V30, V40) was found with the FFF beam for both the IMRT and RA techniques. However, these differences are small too, in most cases less than 1% of the volume of the OAR, especially in comparison to the large standard deviation. The previous study with IMRT and RA showed a minimal improvement in dose to OARs with the FFF beam.[23,26]
Further, after a hysterectomy, the small bowel falls into the pelvis where the uterus previously resided, further increasing the amount of small bowel irradiated to the prescription dose. Rates of grade 2 and higher acute gastrointestinal (GI) toxicity of 50-90% with conventional CRT have been reported in the literature. Acute GI symptoms typically involve varying degrees of diarrhea, cramping, and abdominal pain, which can negatively impact the quality of life during treatment. [14,27] For the bowel, using the FFF beam in both IMRT and RA demonstrates a reduction in dose for V45, which is statistically significant. However, there is an observed increase in dose for V40 with IMRT_FFF and RA_FFF, although this increase is not statistically significant for RA_FFF. Therefore, our study consisted of the study done by Cozzi et al.[28] in which the authors stated that RA showed significant improvements in OAR and normal tissue sparing with uncompromised target coverage compared to IMRT.
Previous studies on the IMRT technique used a PRO optimizer to generate their FF and FFF beam plans.[12,24] Furthermore, sparing plans optimized with PO have higher MLC variability and monitor units for better organs at risk. In the current study, the IMRT and RA plans were generated using the PO optimizer and showed improved sparing of V45 for the bladder in Figure 3 and rectum in Figure 4 with the IMRT technique. Binny et al.[29] showed that IMAT treatment plans with the PO optimizer provide comparable planned dose conformity to target volume and improved OAR sparing compared with the PRO optimizer. Furthermore, studies showed that the PO optimizer generates more complex plans than PRO.[30]
There was a significant increase in MU observed with the FFF beam for IMRT (32.5%, p<0.01) and RA (12.2%, p<0.01) techniques. Though the delivery time of the FFF beam (Dose rate 1440 MU/min) is higher than FF (600 MU/min), the increase in MU is related to achieving uniform dose distribution with an inhomogeneous profile. This finding is in line with the results from previous studies.[31] Furthermore, RA allowed a significant reduction in MUs compared to IMRT, correlating with the risk of increased low-dose normal tissue irradiation, potentially elevating the risk of second malignancies. Therefore, the study of low-dose volume depending upon the treatment technique is essential for inverse planning, as various authors indicate.[32,33] As shown in Figure 5, the RA technique exhibited a decrease in the low-dose volume, with a rapid fall observed with the use of the FFF beam. Additionally, the combined effect of RA and the FFF beam, resulting in a reduction in treatment time, is advantageous in several aspects. It improves patient comfort during treatment, particularly those lying with custom-made masks. It reduces the risk of intra-fraction motion, minimizes organ displacement, and enables the accommodation of more patients for treatment under the same machine.
Conclusion
The advantage of FFF beam planning for post-cervical cancer patients was studied in light of advanced planning techniques with an updated planning platform and various plan indices. The FFF beam achieved a target and OAR dose distribution similar to the FF beam for patients with RA and IMRT plans. RA plans showed significant dosimetric advantages on target coverage and OAR sparing compared with IMRT in treating postoperative cervical cancer with an FFF beam. Additionally, the higher MU for the FFF IMRT plan can be offset by a high dose rate, providing the added benefit of reducing overall treatment time and the motion management of the target.Ethics Committee Approval: The study was approved by the Institutional Ethics Committee (no: IEC/11/65/2023, date: 12/07/2023).
Authorship contributions: Concept - S.M., S.S., P.K.G., R.T.; Design - S.M., S.S., P.K.G., R.T.; Supervision - S.S., P.K.G.; Data collection and/or processing - S.M., R.T.; Data analysis and/or interpretation - S.M., S.S., P.K.G., R.T.; Literature search - S.M., S.S., P.K.G.; Writing - S.M., S.S., P.K.G.; Critical review - S.M., S.S., P.K.G., R.T.
Conflict of Interest: All authors declared no conflict of interest. Use of AI for Writing Assistance: No writing assistance was utilized in the production of this manuscript.
Financial Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Peer-review: Externally peer-reviewed.
References
1) Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram
I, Jemal A, et al. Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality
worldwide for 36 cancers in 185 countries. CA Cancer
J Clin 2021;71(3):209-49.
2) Ong SK, Abe SK, Thilagaratnam S, Haruyama R,
Pathak R, Jayasekara H, et al. Towards elimination of
cervical cancer - Human papillomavirus (HPV) vaccination
and cervical cancer screening in Asian National
Cancer Centers Alliance (ANCCA) member countries.
Lancet Reg Health West Pac 2023;39:100860.
3) Soni S, Pareek P, Manna S, Gayen S, Pundhir A, Tiwari
R, et al. A dosemetric and radiobiological impact of
VMAT and 3DCRT on lumbosacral plexuses, an underestimated
organ at risk in cervical cancer patients.
Rep Pract Oncol Radiother 2022;27(4):624-33.
4) Bai W, Kou C, Yu W, Li Y, Hua W, Yu L, et al. Dosimetric
comparison of volumetric-modulated arc therapy
and intensity-modulated radiation therapy in patients
with cervical cancer: A meta-analysis. Onco Targets
Ther 2018;11:7179-86.
5) Mashhour K, Kamaleldin M, Hashem W. RapidArc vs
conventional IMRT for head and neck cancer irradiation:
Is faster necessary better? Asian Pac J Cancer
Prev 2018;19(1):207-11.
6) Williamson CW, Liu HC, Mayadev J, Mell LK. Advances
in external beam radiation therapy and
brachytherapy for cervical cancer. Clin Oncol (R Coll
Radiol) 2021;33(9):567-78.
7) Chopra S, Gupta S, Kannan S, Dora T, Engineer R,
Mangaj A, et al. Late toxicity after adjuvant conventional
radiation versus image-guided intensity-modulated
radiotherapy for cervical cancer (PARCER):
A randomized controlled trial. J Clin Oncol
2021;39(33):3682-92.
8) Jang H, Park J, Artz M, Zhang Y, Ricci JC, Huh S, et
al. Effective organs-at-risk dose sparing in volumetric
modulated arc therapy using a half-beam technique in
whole pelvic irradiation. Front Oncol 2021;11:611469.
9) Manna S, Kombathula SH, Gayen S, Varshney S, Pareek
P. Dosimetric impact of FFF over FF beam using
VMAT for brain neoplasms treated with radiotherapy.
Polish J Med Phys Eng 2021;27(3):191-9.
10) Mohammed M, Chakir E, Boukhal H, Mroan S, El Bardouni
T. Evaluation of the dosimetric characteristics of
6 MV flattened and unflattened photon beam. Available
at: https://core.ac.uk/download/pdf/82360792.
pdf. Accessed Jun 14, 2024.
11) Manna S, Singh S, Gupta PK, Ragul T. Dosimetric and
radiobiological impact of flattening filter?free beam
and dose calculation algorithm using rapidarc plans
for cervical cancer treatment. Precis Radiat Oncol
2023;7(3):197-206.
12) Tamilarasu S, Saminathan M, Sharma SK, Pahuja A,
Dewan A. Comparative evaluation of a 6MV flattened
beam and a flattening filter free beam for carcinoma of
cervix - IMRT planning study. Asian Pacific J Cancer
Prev 2018;19(3):639-43.
13) Li C, Chen J, Zhu J, Gong G, Tao C, Li Z, et al. Plan
quality comparison for cervical carcinoma treated
with Halcyon and Trilogy intensity-modulated radiotherapy.
J Cancer 2019;10(24):6135-41.
14) Deng X, Han C, Chen S, Xie C, Yi J, Zhou Y, et al. Dosimetric
benefits of intensity-modulated radiotherapy
and volumetric-modulated arc therapy in the treatment
of postoperative cervical cancer patients. J Appl
Clin Med Phys. 2017;18(1):25-31.
15) Collins SP, Coppa ND, Zhang Y, Collins BT, McRae
DA, Jean WC. CyberKnife radiosurgery in the treatment
of complex skull base tumors: Analysis of treatment
planning parameters. Radiat Oncol 2006;1:46.
16) Krishna GS, Srinivas V, Ayyangar KM, Reddy PY.
Comparative study of old and new versions of treatment
planning system using dose volume histogram
indices of clinical plans. J Med Phys 2016;41(3):192-7.
17) Small W Jr, Bosch WR, Harkenrider MM, Strauss JB,
Abu-Rustum N, Albuquerque KV, et al. NRG Oncology/
RTOG consensus guidelines for delineation of
clinical target volume for intensity modulated pelvic
radiation therapy in postoperative treatment of endometrial
and cervical cancer: An update. Int J Radiat
Oncol Biol Phys 2021;109(2):413-24.
18) Paddick I, Lippitz B. A simple dose gradient measurement
tool to complement the conformity index. J Neurosurg
2006;105 Suppl:194-201.
19) Akpati H, Kim C, Kim B, Park T, Meek A. Unified
dosimetry index (UDI): A figure of merit for ranking
treatment plans. J Appl Clin Med Phys 2008;9(3):99-108.
20) Ding C, Chang C, Haslam J, Timmerman R, Solberg
T. SU?FF?T?571: A dosimetric comparison of stereotactic
body radiation therapy irradiation techniques:
Cyberknife versus conventional linac?based systems.
Med Phys 2009;36(6):2656.
21) Sara A, Krabch MEA, Trihi M. Analysis of dosimetric
characteristics of energy 6 MV with and without
flattening filter photon beam generated by the Varian
True Beam linac using Kruskal Wallis H test. Onkol
Radioter 2022;16(5):22-9.
22) Zhang F, Jiang H, Xu W, Wang Y, Gao J, Liu Q, et
al. A dosimetric evaluation of flattening filter-free
volumetric modulated arc therapy for postoperative
treatment of cervical cancer. Oncol Transl Med
2016;2(4):179-84.
23) Treutwein M, Hipp M, Koelbl O, Dobler B. Volumetric-
modulated arc therapy and intensity-modulated
radiation therapy treatment planning for
prostate cancer with flattened beam and flattening
filter free linear accelerators. J Appl Clin Med Phys
2017;18(5):307-14.
24) Kumar L, Kishore V, Bhushan M, Yadav G, Dewan A,
Kumar P, et al. Impact of flattening filter free photon
beam on rapidarc radiotherapy for gynaecological
malignancies: A comparative study. Iran J Med Phys
2021;18(1):23-9.
25) Sharfo AW, Voet PW, Breedveld S, Mens JW, Hoogeman
MS, Heijmen BJ. Comparison of VMAT
and IMRT strategies for cervical cancer patients
using automated planning. Radiother Oncol
2015;114(3):395-401.
26) Kumar L, Yadav G, Samuvel KR, Bhushan M, Kumar
P, Suhail M, et al. Dosimetric influence of filtered and
flattening filter free photon beam on rapid arc (RA) radiotherapy
planning in case of cervix carcinoma. Rep
Pract Oncol Radiother 2017;22(1):10-8.
27) Ray A, Sarkar B. Small bowel toxicity in pelvic radiotherapy
for postoperative gynecological cancer: Comparison
between conformal radiotherapy and intensity
modulated radiotherapy. Asia Pac J Clin Oncol
2013;9(3):280-4.
28) Cozzi L, Dinshaw KA, Shrivastava SK, Mahantshetty
U, Engineer R, Deshpande DD, et al. A treatment
planning study comparing volumetric arc modulation
with RapidArc and fixed field IMRT for cervix uteri
radiotherapy. Radiother Oncol 2008;89(2):180-91.
29) Binny D, Kairn T, Lancaster CM, Trapp JV, Crowe SB.
Photon optimizer (PO) vs progressive resolution optimizer
(PRO): A conformality- and complexity-based
comparison for intensity-modulated arc therapy plans.
Med Dosim 2018;43(3):267-75.
30) Sundaram V, Khanna D, Mohandass P, Vasudeva T.
Comparison of progressive resolution optimizer and
photon optimizer algorithms in RapidArc delivery for
head and neck SIB treatments. Rep Pract Oncol Radiother
2023;28(5):623-35.
31) Ji T, Sun L, Cai F, Li G. Comparison between flattening
filter-free (FFF) and flattened photon beam VMAT
plans for the whole brain radiotherapy (WBRT)
with hippocampus sparing. Asia Pac J Clin Oncol
2022;18(5):e263-7.





