2Radiotherapy Unit, VM Medical Park Kocaeli Hospital, Kocaeli-Türkiye
3Department of Biomedical Engineering, Kocaeli University, Kocaeli-Türkiye DOI : 10.5505/tjo.2024.4332
Summary
OBJECTIVEIn this study, different fraction sizes in prostate cancer will be examined using a radiobiological model.
METHODS
Fifty patients diagnosed with low-risk prostate cancer who were treated between 2009 and 2021 were
identified retrospectively. Conventional (CF) and ultra-hypofractionated (HF) volume-adjusted arc plans
were calculated for selected prostate patients. Mathematical formulations were created for radiobiological
modeling of tumor control probability (TCP) and normal tissue complication probability (NTCP) in the
Matlab program. Dose-volume histogram (DVH) data of the plans were examined in the Matlab program.
RESULTS
Bladder and rectum NTCP values were significantly lower in the HF technique compared to the CF technique
(p<0.05). For femoral heads, NTCP was similar (p=0.317). For the planned target volume, TCP
values obtained with the HF technique are significantly higher compared to the CF technique (p=0.000).
CONCLUSION
Higher TCP values were obtained with the HF technique. It has been observed that normal tissues receive
lower doses. This may be associated with high local control rates while providing similar toxicity.
HF treatment may be preferred in prostate cancer because the total treatment duration is shorter and
the dose applied to critical organs is lower. Radiobiological models are very instructive for comparing
treatment schemes in radiotherapy plans. The Matlab program we created is a very helpful tool for the
radiation oncologist and medical physicist to evaluate their plans.
Introduction
Radiotherapy is one of the main treatment methods in cancer treatment. The aim of radiotherapy is to give as low radiation as possible to the surrounding healthy tissues while giving a high dose to the cancerous tissue. For this reason, tolerance doses for each organ should be followed, and these doses should not be exceeded during the treatment phase. In order for the cancerous cell to respond to radiation, it is important to give an excessive dose that can damage the cell nucleus and, at the same time, not exceed the tolerance dose of neighboring healthy tissues in terms of not losing organ function. Intensive research has been carried out to increase the radiation dose for more effective treatments. To ensure tumor treatment efficacy, the hypofractionated treatment method was tried to be completed at a higher dose than conventional fractionation and in a shorter time than conventional fractionation.[1]Fractionation types vary according to the reduction of normal tissue toxicity while giving the necessary dose to control the cancer cell. As a result of clinical experience, the type of fractionation in which tumor control is achieved without losing the function of normal tissues? a daily tumor dose of 1.8?2 Gy is applied five times a week?is called conventional fractionation. As the fraction dose applied to the tumor exceeds 2 Gy, the tumor damage rate increases. Radiotherapy is one of the frequently preferred treatment methods in the treatment of prostate cancer. Numerous studies have been carried out around the world for more effective radiation doses in the last 20 years, which have seen the radiation doses for these diseases being changed, especially in recent years. It has been shown that switching to a hypofractionated dose in prostate cancer increases the efficacy of treatment on the tumor, reducing the side effects, hence leading to cost-effective treatment.[1]
Success in clinical radiotherapy is related to radiation dose. Low doses have little ability to destroy the tumor. Local disease control can be achieved at high doses. There is a sigmoidal relationship between the probability of tumor control and the dose. For any type of cancer, this curve is associated with treatment success. The dose-response curve depends on some biological factors such as the time elapsed after radiotherapy, the duration of the radiation dose, the volume of irradiated normal tissue, and the quality of the beam used for radiotherapy in relation to the response of the tissues to radiation.[2] As the dose increases, the probability of tumor control increases, but this also increases the probability of normal tissue complications. The dose that provides both conditions is the optimal treatment dose. Treatment protocols with a daily fraction dose greater than 2 Gy and a fraction number of 20 or less are called hypofractionated therapy.[1]
Preventing various complications can be achieved by limiting the dose to which normal tissues will be exposed. The dose that can control half of the tumor volume is called TCD50, and the TCD50 value and the slope of the dose-response curve are important.[3] For critical organs surrounding the tumor, biological advantage can be achieved by increasing the dose per fraction with the hypofractionated scheme.[4]
Conventional dose-volume histograms do not compare different fractionation schemes, hence making it difficult to compare plans with conventional DVH.[5] For this reason, the comparison of the plan with the TCP/NTCP model is important. Its use in commercial planning systems is not common due to the uncertainties in the parameters used in radiobiological models.[6]
The linear quadratic model (LQ) model is commonly used in fractionated external radiotherapy to describe the dose response to the survival of cells in the irradiated volume. TCP/NTCP curves can be used to compare and select the best plan for treatment.[5,7] Lyman developed the NTCP model for a partially irradiated organ in 1985.[8]
Clinicians have to rely on the DVH characteristics of different tissues when evaluating a plan. The TCP/ NTCP radiobiological model uses clinical data based on the dose-volume characteristics of different tissues. Radiobiological modeling plays an important role in the creation of the treatment plan and in the optimization process. The radiobiological model of TCP is a measure of success in treatment.[7,9]
TCP and NTCP models need tissue-specific parameters and NTCP curves have 95% reliability. Commonly used NTCP models are the Lyman et al.[8] Relative Seriality (RS) model.[10]
Biological optimization is a treatment plan that takes into account radiation-related biological parameters, including the possibility of tumor control and the possibility of complications that may occur in normal tissue.[11] Work on TCP and NTCP began in the 1980s and the first half of the 1990s. These models have been limited to evaluating the treatment plan over time. The tumor can be brought under control by killing each tumor clonogen (cells with the potential for uncontrolled division) cell. This is explained by the Poisson statistics. Lack of information on cell radiosensitivity and clonogeny is one of the main reasons why TCP models are not used in the clinic. Organs are not homogeneous within themselves. For example, the apex of the lung is less sensitive than its other parts. This situation causes differences between plans for NTCP.[2,11] The response to radiation therapy is a multifaceted phase. The control of the tumor and the possibility of complications in normal tissues must be determined with absolute accuracy, and the defined dose can be adjusted individually. It is necessary to take into account factors affecting dose and different responses to radiation. Mixing different data types with each other and reflecting them on the treatment is possible with machine learning by correctly introducing TCP and NTCP concepts into planning systems. This is not yet widely used in current treatment planning systems.[12]
Matlab programming language is used in many areas of engineering and science. A matrix-based calculation language is used. It has a user-friendly interface and is practical to use. It is a program that can also be integrated into other programming languages.[13]
This study aims to investigate the most appropriate treatment technique for clinical use by examining the effects of conventional and ultra-hypofractionated treatment schemes on normal tissue complications and the possibility of tumor control using the equivalent uniform dose (EUD)-based radiobiological model created in Matlab.
Methods
Based on power analysis, 50 patients with localized prostate cancer were identified retrospectively.[14,15] Data were obtained for the treatment of patients diagnosed with prostate cancer and treated with radiotherapy in our center between 2009 and 2021. Siemens Emotion Duo (Siemens Healthcare, Erlangen, Germany) computed tomography (CT) simulation images were anonymized. The prostate bed was determined as the clinical target volume (CTV). PTV was created by giving a 10 mm margin to the CTV. The same delineated contours were used for both CF and HF plans for an accurate benchmark. The plan acceptance criterion was determined as 100% of the prescription dose covering at least 95% of the target volume. The critical organ dose limits for HF and CF treatment methods are shown in Table 1 and Table 2. The volumetric modulated arc therapy (VMAT) technique was used for the ultra-hypofractionated (HF) and conventional (CF) treatment schemes in prostate treatment.[16-18]Table 1 Prostate HF critical organ dose limits
Table 2 Prostate CF critical organ dose limits
Treatment plans were performed using 2 arc treatment fields with 6 MV x-ray energy in the Eclipse V13.6 (Varian Medical Systems, Palo Alto, CA) treatment planning system. The first full arc angle was determined as 181?179 degrees in the clockwise direction, and the second full arc was determined as 179-181 degrees in the counterclockwise direction. A 30-degree collimator angle was used to minimize leaf leakage between two arcs. In Figure 1, the isodoses received by the HF and CF plans converted to 2 Gy equivalent doses are compared. As seen in Figure 1, in the HF plan there are fewer low-dose zones and also less 50% isodose on the rectum. D15, D25, D35, and D50 and the mean dose values specified in RTOG 0415 were used to compare rectal and bladder doses when evaluating different dose schemes in prostate cancer.[4]
HF and CF plan DVH is shown in Figure 2. With HF planning, less bladder and rectum doses, as well as more PTV doses, appear to be on DVH. The femoral heads appear to receive more doses in the low-dose region with the HF treatment technique.
For prostate cancer patients, a total of 5 fractions of 6.7 Gy/fraction/every other day for HF and a total of 39 fractions of 2 Gy/fraction/day were used as the conventional scheme.[15] Treatment plans were made with the anisotropic analytical algorithm (AAA) algorithm. DVH was examined in the acquired plans. Organ-specific α/β values were used.[19] The relation between biological equivalent dose (BED) and 2 Gy equivalent dose (EQD2), which is used to change the dose equivalent to 2 Gy between HF treatment and CF treatment, is as in Equation 1 and Equation 2.[20] The equations we used are as follows:

Di fraction dose, n is the number of fractions.
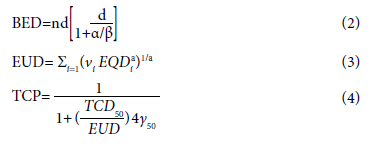
a and γ are the unitless model parameters for normal and target volume. vi is the volume that receives the Di dose in its partial volume. nf is the number of fractions. α/β is a tissue-specific parameter according to the linear quadratic model. In radiotherapy, TCP ≥0.5 and NTCP ≤0.05 are generally preferred.[21]
EUD gives information about equivalent doses that produce the same biological effect between two dose distributions. 2 Gy radiation dose is a parameter that represents the clonogen number and sensitivity to radiation.[9]
Gay and Niemerko calculated the NTCPGN according to the mean lung dose.[21] Accordingly, the NTCPMLD account is as follows:
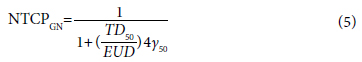
In Equation 3, Equation 4, and Equation 5, Vi is the volume of the organ that receives the Di dose, and a=1/n is the tissue-specific partial volume parameter. TD50 is the dose that causes 50% damage when the relevant organ is irradiated homogeneously.
There are programs in the literature for TCP and NTCP calculations.[21] Niemerko used the Matlab programming language to calculate the EUD model TCP-NTCP.[22] The DVH data obtained from the treatment planning system were transferred to the Matlab program in American Standard Code for Information Interchange (ASCII) format. Table 3 gives the parameters used in prostate cancer for Niemerko's EUD-based radiobiological model.[21,23]
Table 3 NTCP radiobiological model critical organ values for prostate cancer
In our study, we used Equations 1, 2, 3, 4, and 5 for TCP and NTCP evaluation. Cumulative DVH data were calculated for PTV, bladder, rectum, and femoral heads in HF and CF prostate cancer plans.[22,24]
The Statistical Package for the Social Sciences (SPSS) V25 program was used for statistical analysis. Whether the data showed normal distribution was examined with the Kolmogorov-Smirnov test. Paired ttest was applied to normally distributed data sets, and Wilcoxon matched-pair signed-rank test was applied to data sets that did not show normal distribution. Values below p<0.05 were considered significant based on the alpha error rate of 5%.
Results
Table 4 shows the doses received by the rectum, bladder, and femoral heads in accordance with the stereotactic and conventional fractionation for prostate cancer. Rectal dose-volume statistics were shown to be statistically lower with stereotactic radiotherapy. The dose-volume histogram data of the bladder in conventional and hypofractional therapy did not show any significant differences; however, the application of conventional fractionation resulted in lower doses to the femoral heads.Table 4 Dosimetric parameters of critical organs for prostate cancer
The radiobiological effects of hypofractional and conventional treatment techniques on low-risk prostate cancers were shown in Table 5. There was a significant difference in TCP values in favor of hypofractionated treatment. EUD values were also significantly higher for PTV in hypofractionated planning.
Table 5 Tumor control probability (TCP), equivalent uniform dose (EUD) results for prostate cancer
As shown in Table 6, the probability of normal tissue complications in both treatment modalities is the same, even though EUD for the femoral heads in hypofractionated treatment is higher than in conventional treatment in the low-dose region.
Table 6 Normal Tissue Complication Probability (NTCP) and equivalent uniform dose results
It was discovered that the bladder EUD and NTCP values were significantly lower in hypofractional treatment. The NTCP value was computed lower in the hypofractionated treatment technique, despite the fact that the rectum EUD values were similar in both treatment approaches.
Discussion
In this study, we have compared the conventional fractionation (CF) and ultra-hypofractionated (HF) radiotherapy techniques in the treatment of low-risk prostate cancer. This comparison focused on the tumor control probability (TCP) and normal tissue complication probability (NTCP) using radiobiological models. Radiobiological models were created in the Matlab programming language. Factors TCD50, TD50, γ, and aaa explained in the methods section were used for TCP and NTCP radiobiological model calculations.[25]Nuraini et al.[25] found that normal tissue cells were repaired 15 hours after irradiation. When the time between fractions is less than 15 hours, normal tissue damage will increase. At the same time, they examined using TCP and NTCP models that the irradiation time should be kept short in order to increase tumor damage. This emphasizes the importance of fraction size. Consistent with this study, we found our TCP & NTCP data to be significantly different in favor of HF plans.
Mesbahi et al.[26] compared 3D conformal and intensity-modulated radiotherapy plans for prostate cancer using radiobiological models. They used Poisson, EUD, and Lyman-Kutcher-Burman radiobiological models for TCP and NTCP calculations. Radiobiologically, they observed that critical organs were better protected in intensity-modulated radiotherapy (IMRT) plans. It is more preferable due to the clinical results of HF treatment, because a higher dose could be delivered to the tumor in a short treatment time.[27,28] Schell et al.[5] compared hypofractionated (HF) and conventional fractionation (CF) radiotherapy in prostate cancer, demonstrating consistent findings regarding the benefits of HF. However, in clinical practice, it is very important to carefully evaluate parameter values to prevent the risks of inadequate dosage. Consistent with our study, it addresses the importance of precision in treatment planning while acknowledging the benefits of HF. Schell et al.[5] when they compared the plans prepared with prostate HF and CF doses with the treatment planning system using the radiobiological model, observed that there was no significant difference between the doses received by the target volume, and normal tissues received less doses in the HF treatment.
The HF technique demonstrated significantly higher TCP values compared to the CF technique, indicating a greater likelihood of tumor control with the HF regimen. This is attributed to the higher doses administered to the tumor over fewer fractions, which intensifies the radiation effect while reducing overall treatment time. These findings align with the results of other studies, such as those conducted by Clemente-Gutiérrez et al.[24] and Sukhih et al.[29] which have also emphasized the effectiveness of HF regimens in increasing TCP and reducing NTCP values for normal tissues.
Our results show that the NTCP values for bladder and rectum were significantly lower in the HF plans compared to CF plans, suggesting that HF is less likely to cause complications in these tissues. This is consistent with Mesbahi et al.[26]'s findings, which indicated better protection of critical organs with intensity-modulated radiotherapy (IMRT) plans. The femoral head NTCP values did not show significant differences between the two techniques, suggesting comparable safety profiles in this regard.[20]
The HF technique's higher TCP and lower NTCP values for critical organs indicate that this method may offer superior clinical outcomes compared to CF. HF treatment, with its shorter overall duration, is more convenient for patients and may result in fewer side effects. This aspect of patient comfort and reduced treatment burden is crucial in clinical settings.
Clemente-Gutiérrez et al.[24] conducted a study on the use of biological parameters in treatment plan quality control, where they stated that radiobiological models are an alternative method for evaluating pretreatment plans.
The use of radiobiological models proves invaluable in comparing different treatment schemes and should be a standard component of radiotherapy planning systems.[26,28] As a result of comparing hypofractionated and conventional dose treatment techniques, higher TCP and EUD values were obtained with hypofractionated treatment. Low NTCP and EUD values were obtained with the hypofractionated treatment technique for prostate cancer normal tissues. Radiobiological models are very instructive for comparing treatment schemes in radiotherapy plans. When we compare the hypofractionated dose with the conventional dose for prostate cancer, the doses obtained are within acceptable limits. Significant reductions in rectal doses were obtained with hypofractionation. Since SBRT treatment takes a shorter time, it is more advantageous than conventional fractionation in terms of patient comfort.
Conclusion
The HF technique's higher TCP and lower NTCP values for critical organs indicate that this method may offer superior clinical outcomes compared to CF. HF treatment, with its shorter overall duration, is more convenient for patients and may result in fewer side effects. This aspect of patient comfort and reduced treatment burden is crucial in clinical settings.While our study provides compelling evidence supporting the use of HF in low-risk prostate cancer, it is essential to consider the limitations associated with radiobiological modeling. Factors such as individual patient characteristics, tissue-specific responses, and other treatment variables like chemotherapy and surgery must be accounted for in future studies. Integrating comprehensive radiobiological models into clinical practice will enhance treatment planning and outcomes.
High fraction doses are especially suggestive for normal tissue complications compared to standard fraction doses. Since many factors, such as NTCP modeling, patient characteristics, cell structure, chemotherapy, and surgery, affect toxicity, studies should be conducted to include all parameters. Radiobiological models are needed for safer and more curative treatments at high doses. Since it is difficult to compare different plans with normal DVH, radiobiological model parameters should be integrated into today's planning systems in order to determine the damage to the tissue, and treatment plans should be created with these parameters.
The study's findings underscore the potential benefits of HF radiotherapy in achieving higher tumor control with fewer complications compared to conventional fractionation. These insights are pivotal for optimizing prostate cancer treatment protocols and improving patient quality of life.
Ethics Committee Approval: The study was approved by the Kocaeli University Non-Interventional Clinical Research Ethics Committee (no: 2021/265, date: 16/12/2021).
Authorship contributions: Concept - A.Ü.K., M.S.K., M.G.A., Ö.G.; Design - A.Ü.K., B.T., G.Ö., A.O.K.; Supervision - A.Ü.K.; Funding - M.G.A., Ö.G.; Materials - A.Ü.K., B.T., G.Ö., A.O.K., U.D.; Data collection and/or processing - A.Ü.K.; Data analysis and/or interpretation - A.Ü.K., U.D., M.S.K., M.G.A., Ö.G.; Literature search - A.Ü.K., B.T., G.Ö., A.O.K.; Writing - A.Ü.K.; Critical review - A.Ü.K., U.D., M.S.K., M.G.A., Ö.G.
Conflict of Interest: All authors declared no conflict of interest. Use of AI for Writing Assistance: Not declared.
Financial Support: None declared.
Peer-review: Externally peer-reviewed.
References
1) Bilge Becerir H. Radyoterapi fiziği. Ankara: Nobel
Akademik Yayıncılık; 2020.
2) Mayles P, Nahum A, Rosenwald JC. Handbook of radiotherapy
physics: Theory and practice. Oxfordshire:
Taylor & Francis; 2007.
3) Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation
dose-response of human tumors. Int J Radiat Oncol
Biol Phys 1995;32(4):1227-37.
4) Hernández TG, González AV, Peidro JP, Ferrando JV,
González LB, Cabañero DG, et al. Radiobiological
comparison of two radiotherapy treatment techniques
for high-risk prostate cancer. Rep Pract Oncol Radiother
2013;18(5):265-71.
5) Schell S, Wilkens JJ, Oelfke U. Radiobiological effect
based treatment plan optimization with the linear
quadratic model. Z Med Phys 2010;20(3):188-96.
6) Allen Li X, Alber M, Deasy JO, Jackson A, Ken Jee
KW, Marks LB, et al. The use and QA of biologically
related models for treatment planning: Short report of
the TG-166 of the therapy physics committee of the
AAPM. Med Phys 2012;39(3):1386-409.
7) Bruzzaniti V, Abate A, Pedrini M, Benassi M, Strigari
L. IsoBED: A tool for automatic calculation of biologically
equivalent fractionation schedules in radiotherapy
using IMRT with a simultaneous integrated boost
(SIB) technique. J Exp Clin Cancer Res 2011;30(1):52.
8) Lyman JT. Complication probability as assessed from
dose-volume histograms. Radiat Res. 1985;104(2):13-9.
9) Warkentin B, Stavrev P, Stavreva N, Field C, Fallone
BG. A TCP-NTCP estimation module using DVHs
and known radiobiological models and parameter
sets. J Appl Clin Med Phys 2004;5(1):50-63.
10) Zhang L, Hub M, Thieke C, Floca RO, Karger CP. A
method to visualize the uncertainty of the prediction
of radiobiological models. Phys Med 2013;29(5):556-61.
11) Nahum AE, Uzan J. (Radio)biological optimization of
external-beam radiotherapy. Comput Math Methods
Med 2012;2012:329214.
12) Gulliford S, El Naqa I. Modelling of Radiotherapy Response
(TCP/NTCP). In: El Naqa I, Murphy MJ, editors.
Machine and deep learning in oncology, medical
physics and radiology. 2nd ed. New York: Springer;
2022. p. 399-437.
13) MathWorks. MATLAB onramp. Available from:
https://www.mathworks.com/learn/tutorials/matlabonramp.
html. Accessed Jun 14, 2024.
14) Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn
PP, de Jager H, et al. Hypofractionated versus conventionally
fractionated radiotherapy for patients with
prostate cancer (HYPRO): Late toxicity results from a
randomised, non-inferiority, phase 3 trial. Lancet Oncol
2016;17(4):464-74.
15) Lan KKG, DeMets DL. Discrete sequential boundaries
for clinical trials. Biometrika 1983;70(3):659?63.
16) Pocock SJ. Group sequential methods in the design and
analysis of Clinical Trials. Biometrika 1977;64(2):191-9.
17) Murray LJ, Lilley J, Thompson CM, Cosgrove V, Mason
J, Sykes J, et al. Prostate stereotactic ablative radiation
therapy using volumetric modulated arc therapy
to dominant intraprostatic lesions. Int J Radiat Oncol
Biol Phys 2014;89(2):406-15.
18) Expert Panel on Radiation Oncology-Prostate:;
Zaorsky NG, Showalter TN, Ezzell GA, Nguyen PL,
Assimos DG, D'Amico AV, et al. ACR Appropriateness
Criteria® external beam radiation therapy treatment
planning for clinically localized prostate cancer, part I
of II. Adv Radiat Oncol 2016;2(1):62-84.
19) Jones B, Dale RG, Deehan C, Hopkins KI, Morgan
DA. The role of biologically effective dose (BED)
in clinical oncology. Clin Oncol (R Coll Radiol)
2001;13(2):71-81.
20) Chang JH, Gehrke C, Prabhakar R, Gill S, Wada M,
Lim Joon D, et al. RADBIOMOD: A simple program
for utilising biological modelling in radiotherapy plan
evaluation. Phys Med 2016;32(1):248-54.
21) Kong FM, Pan C, Eisbruch A, Ten Haken RK.
Physical models and simpler dosimetric descriptors
of radiation late toxicity. Semin Radiat Oncol
2007;17(2):108-20.
22) Gay HA, Niemierko A. A free program for calculating
EUD-based NTCP and TCP in external beam radiotherapy.
Phys Med 2007;23(3-4):115-25.
23) Rana S, Cheng C. Radiobiological impact of planning
techniques for prostate cancer in terms of tumor control
probability and normal tissue complication probability.
Ann Med Health Sci Res 2014;4(2):167-72.
24) Clemente-Gutiérrez F, Pérez-Vara C, Clavo-Herranz
MH, López-Carrizosa C, Pérez-Regadera J, Ibáñez-
Villoslada C. Assessment of radiobiological metrics applied
to patient-specific QA process of VMAT prostate
treatments. J Appl Clin Med Phys 2016;17(2):341-67.
25) Nuraini, R., &Widita, R. (2019). Tumor control probability
(TCP) and normal tissue complication probability
(NTCP) with consideration of cell biological effect.
J Phys Conf, 1245(1), 012092.
26) Mesbahi A, Rasouli N, Mohammadzadeh M, Nasiri
Motlagh B, Ozan Tekin H. Comparison of radiobiological
models for radiation therapy plans of prostate
cancer: Three-dimensional conformal versus intensity
modulated radiation therapy. J Biomed Phys Eng
2019;9(3):267-78.
27) Ray KJ, Sibson NR, Kiltie AE. Treatment of breast and
prostate cancer by hypofractionated radiotherapy: Potential risks and benefits. Clin Oncol (R Coll Radiol)
2015;27(7):420-6.





